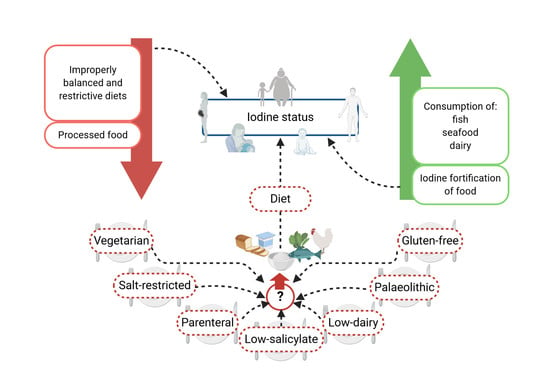
Is There an Ideal Diet to Protect against Iodine Deficiency?
Abstract
:1. Introduction
2. Iodine Deficiency
2.1. Iodine Deficiency among Various Populations
2.2. Health Consequences of Iodine Deficiency
2.3. Iodine Deficiency and Breast Cancer
2.4. Iodine Deficiency in Pregnant Women
3. Iodine Recommended Intake
3.1. Iodine Intake from the Diet, Iodine Fortification and Recommendations
3.2. Food Sources of Iodine
4. Diets and a Reduced Iodine Intake
4.1. Hypertension and Salt-Restrictive Diet
4.2. Vegan and Vegetarian Diets
4.3. Gluten-Free Diet
4.4. Iodine Intake and Dairy Foods
4.5. Parenteral Nutrition
4.6. Palaeolithic Diet
4.7. Low-Salicylate Diet
5. Excessive Intake of Iodine
5.1. Excessive Intake of Iodine among Various Populations
5.2. Pharmacotherapy and Excessive Iodine Intake
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ingbar, S.H. Autoregulation of the Thyroid. Response to Iodide Excess and Depletion. Mayo Clin. Proc. 1972, 47, 814–823. [Google Scholar]
- Pisarev, M.A. Thyroid Autoregulation. J. Endocrinol. Investig. 1985, 8, 475–484. [Google Scholar] [CrossRef]
- O’Kane, S.M.; Mulhern, M.S.; Pourshahidi, L.K.; Strain, J.J.; Yeates, A.J. Micronutrients, Iodine Status and Concentrations of Thyroid Hormones: A Systematic Review. Nutr. Rev. 2018, 76, 418–431. [Google Scholar] [CrossRef]
- Pironi, L.; Guidetti, M.; Agostini, F. Iodine Status in Intestinal Failure in Adults. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Zbigniew, S. Iodine Prophylaxis in the Lights of the Last Recommendation of WHO on Reduction of Daily Salt Intake. Recent Pat. Endocr. Metab. Immune Drug Discov. 2017, 11, 39–42. [Google Scholar] [CrossRef]
- Santos, J.A.R.; Christoforou, A.; Trieu, K.; McKenzie, B.L.; Downs, S.; Billot, L.; Webster, J.; Li, M. Iodine Fortification of Foods and Condiments, Other than Salt, for Preventing Iodine Deficiency Disorders. Cochrane Database Syst. Rev. 2019, 2, CD010734. [Google Scholar] [CrossRef] [PubMed]
- Angermayr, L.; Clar, C. Iodine Supplementation for Preventing Iodine Deficiency Disorders in Children. Cochrane Database Syst. Rev. 2004, CD003819. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Andersson, M.; Zimmermann, M.B. Global Iodine Nutrition: Where Do We Stand in 2013? Thyroid 2013, 23, 523–528. [Google Scholar] [CrossRef]
- Carlsen, M.H.; Andersen, L.F.; Dahl, L.; Norberg, N.; Hjartåker, A. New Iodine Food Composition Database and Updated Calculations of Iodine Intake among Norwegians. Nutrients 2018, 10, 930. [Google Scholar] [CrossRef] [Green Version]
- Pyka, B.; Zieleń-Zynek, I.; Kowalska, J.; Ziółkowski, G.; Hudzik, B.; Gąsior, M.; Zubelewicz-Szkodzińska, B. Iodine Dietary Recommendations- in Search of a Consensus between Cardiologists and Endocrinologists. Folia Cardiol. 2019, 14, 156–160. [Google Scholar] [CrossRef]
- Pearce, E.N.; Lazarus, J.H.; Moreno-Reyes, R.; Zimmermann, M.B. Consequences of Iodine Deficiency and Excess in Pregnant Women: An Overview of Current Knowns and Unknowns. Am. J. Clin. Nutr. 2016, 104 (Suppl. 3), 918S–923S. [Google Scholar] [CrossRef] [Green Version]
- The Iodine Global Network. Global Scorecard of Iodine Nutrition in 2020 in the General Population Based on School-Age Children (SAC); IGN: Ottawa, ON, Canada, 2020. [Google Scholar]
- De Benoist, B.; McLean, E.; Andersson, M.; Rogers, L. Iodine Deficiency in 2007: Global Progress since 2003. Food Nutr. Bull. 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, E.M.; Sullivan, K.M.; Perrine, C.G.; Rogers, L.M.; Peña-Rosas, J.P. Comparison of Median Urinary Iodine Concentration as an Indicator of Iodine Status among Pregnant Women, School-Age Children, and Nonpregnant Women. Food Nutr. Bull. 2011, 32, 206–212. [Google Scholar] [CrossRef]
- Panth, P.; Guerin, G.; DiMarco, N.M. A Review of Iodine Status of Women of Reproductive Age in the USA. Biol. Trace Elem. Res. 2019, 188, 208–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adalsteinsdottir, S.; Tryggvadottir, E.A.; Hrolfsdottir, L.; Halldorsson, T.I.; Birgisdottir, B.E.; Hreidarsdottir, I.T.; Hardardottir, H.; Arohonka, P.; Erlund, I.; Gunnarsdottir, I. Insufficient Iodine Status in Pregnant Women as a Consequence of Dietary Changes. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef] [Green Version]
- Gizak, M.; Rogers, L.; Gorstein, J.; Zimmermann, M.; Andersson, M. Global Iodine Status in School-Age Children, Women of Reproductive Age, and Pregnant Women in 2017. In Proceedings of the Presented as a poster at Nutrition 2018, the American Society for Nutrition Annual Conference, Boston, MA, USA, 9–12 June 2018. [Google Scholar]
- Gietka-Czernel, M. Iodine Prophylaxis. Postępy Nauk Med. 2015, XXVIII, 839–845. [Google Scholar]
- Williams, E.D. Dietary iodide and thyroid cancer. In Thyroid Disorders Associated with Iodine Deficiency and Excess; Hall, R., Köbberling, J., Eds.; Raven Press: New York, NY, USA, 1985; pp. 201–207. [Google Scholar]
- Dijkstra, B.; Prichard, R.S.; Lee, A.; Kelly, L.M.; Smyth, P.P.A.; Crotty, T.; McDermott, E.W.; Hill, A.D.K.; O’Higgins, N. Changing Patterns of Thyroid Carcinoma. Ir. J. Med. Sci 2007, 176, 87–90. [Google Scholar] [CrossRef]
- Harach, H.R.; Escalante, D.A.; Onativia, A.; Lederer Outes, J.; Saravia Day, E.; Williams, E.D. Thyroid Carcinoma and Thyroiditis in an Endemic Goitre Region before and after Iodine Prophylaxis. Acta Endocrinol. 1985, 108, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Moses, W.; Rahbari, R.; Khanafshar, E.; Duh, Q.-Y.; Clark, O.; Kebebew, E. Higher Rate of BRAF Mutation in Papillary Thyroid Cancer over Time: A Single-Institution Study. Cancer 2011, 117, 4390–4395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskin, B.A. Iodine Metabolism and Breast Cancer. Trans. N. Y. Acad. Sci. 1970, 32, 911–947. [Google Scholar] [CrossRef]
- Stadel, B.V. Dietary Iodine and Risk of Breast, Endometrial, and Ovarian Cancer. Lancet 1976, 1, 890–891. [Google Scholar] [CrossRef]
- Malya, F.U.; Kadioglu, H.; Hasbahceci, M.; Dolay, K.; Guzel, M.; Ersoy, Y.E. The Correlation between Breast Cancer and Urinary Iodine Excretion Levels. J. Int. Med. Res. 2018, 46, 687–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappaport, J. Changes in Dietary Iodine Explains Increasing Incidence of Breast Cancer with Distant Involvement in Young Women. J. Cancer 2017, 8, 174–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venturi, S. Is There a Role for Iodine in Breast Diseases? Breast 2001, 10, 379–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, P.P.A. Role of Iodine in Antioxidant Defence in Thyroid and Breast Disease. Biofactors 2003, 19, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, C.; Rovera, F.; Rausei, S.; Del Ferraro, S.; Fachinetti, A.; Lavazza, M.; Marchionini, V.; Arlant, V.; Tanda, M.L.; Piantanida, E.; et al. Breast Cancer and Thyroid Diseases: Analysis of 867 Consecutive Cases. J. Endocrinol. Investig. 2017, 40, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Chang, Y.; Lee, K.H.; Yun, J.-S.; Park, Y.L.; Park, C.H.; Ahn, J.; Shin, H.; Ryu, S. Serum Concentration of Thyroid Hormones in Abnormal and Euthyroid Ranges and Breast Cancer Risk: A Cohort Study. Int. J. Cancer 2019, 145, 3257–3266. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulos, D.A.; Kapolla, N.; Michalakis, A.; Vassilaros, S.; Papadiamantis, J.; Georgiakodis, F. Thyroid Disease in Patients with Benign and Malignant Mastopathy. Cancer 1986, 57, 125–128. [Google Scholar] [CrossRef]
- Turken, O.; NarIn, Y.; DemIrbas, S.; Onde, M.E.; Sayan, O.; KandemIr, E.G.; YaylacI, M.; Ozturk, A. Breast Cancer in Association with Thyroid Disorders. Breast Cancer Res. 2003, 5, R110–R113. [Google Scholar] [CrossRef]
- Gogas, J.; Kouskos, E.; Tseleni-Balafouta, S.; Markopoulos, C.; Revenas, K.; Gogas, G.; Kostakis, A. Autoimmune Thyroid Disease in Women with Breast Carcinoma. Eur. J. Surg. Oncol. 2001, 27, 626–630. [Google Scholar] [CrossRef]
- Smyth, P.P. The Thyroid, Iodine and Breast Cancer. Breast Cancer Res. 2003, 5, 235–238. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Wang, B.; Lu, X.; Miao, S.; Yang, F.; Zava, T.; Ding, Q.; Zhang, S.; Liu, J.; Zava, D.; et al. Iodine Stimulates Estrogen Receptor Singling and Its Systemic Level Is Increased in Surgical Patients Due to Topical Absorption. Oncotarget 2018, 9, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Levie, D.; Korevaar, T.I.M.; Bath, S.C.; Murcia, M.; Dineva, M.; Llop, S.; Espada, M.; van Herwaarden, A.E.; de Rijke, Y.B.; Ibarluzea, J.M.; et al. Association of Maternal Iodine Status With Child IQ: A Meta-Analysis of Individual Participant Data. J. Clin. Endocrinol. Metab. 2019, 104, 5957–5967. [Google Scholar] [CrossRef] [Green Version]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of Inadequate Iodine Status in UK Pregnant Women on Cognitive Outcomes in Their Children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef]
- Gietka-Czernel, M.; Dębska, M.; Kretowicz, P.; Jastrzębska, H.; Kondracka, A.; Snochowska, H.; Ołtarzewski, M. Iodine Status of Pregnant Women from Central Poland Ten Years after Introduction of Iodine Prophylaxis Programme. Endokrynol. Pol. 2010, 61, 646–651. [Google Scholar] [PubMed]
- Hetzel, B.S. Iodine Deficiency Disorders (IDD) and Their Eradication. Lancet 1983, 2, 1126–1129. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-Deficiency Disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef]
- Cui, T.; Wang, W.; Chen, W.; Pan, Z.; Gao, S.; Tan, L.; Pearce, E.N.; Zimmermann, M.B.; Shen, J.; Zhang, W. Serum Iodine Is Correlated with Iodine Intake and Thyroid Function in School-Age Children from a Sufficient-to-Excessive Iodine Intake Area. J. Nutr. 2019, 149, 1012–1018. [Google Scholar] [CrossRef]
- Charlton, K.; Probst, Y.; Kiene, G. Dietary Iodine Intake of the Australian Population after Introduction of a Mandatory Iodine Fortification Programme. Nutrients 2016, 8, 701. [Google Scholar] [CrossRef] [Green Version]
- Maalouf, J.; Barron, J.; Gunn, J.P.; Yuan, K.; Perrine, C.G.; Cogswell, M.E. Iodized Salt Sales in the United States. Nutrients 2015, 7, 1691–1695. [Google Scholar] [CrossRef] [Green Version]
- Charlton, K.; Skeaff, S. Iodine Fortification: Why, When, What, How, and Who? Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Office of Dietary Supplements-Iodine. Available online: https://ods.od.nih.gov/factsheets/Iodine-HealthProfessional/ (accessed on 21 July 2020).
- Russell, R.M. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, WA, USA, 2001. [Google Scholar]
- Krzepilko, A.; Zych-Wezyk, I.; Molas, J. Alternative Ways of Enriching the Human Diet with Iodine. J. Pre-Clin. Clin. Res. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Fuge, R.; Johnson, C.C. The Geochemistry of Iodine-a Review. Env. Geochem. Health 1986, 8, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Dellavalle, M.E.; Barbano, D.M. Iodine Content of Milk and Other Foods. J. Food Prot. 1984, 47, 678–684. [Google Scholar] [CrossRef]
- Flachowsky, G.; Franke, K.; Meyer, U.; Leiterer, M.; Schöne, F. Influencing Factors on Iodine Content of Cow Milk. Eur. J. Nutr. 2014, 53, 351–365. [Google Scholar] [CrossRef]
- Köhler, M.; Fechner, A.; Matthias, L.; Spörl, K.; Remer, T.; Schäfer, U.; Jahreis, G. Iodine Content in Milk from German Cows and in Human Milk: New Monitoring Study. Trace. Elem. Electrolytes 2012, 29, 119–126. [Google Scholar] [CrossRef] [Green Version]
- O’Kane, S.M.; Pourshahidi, L.K.; Mulhern, M.S.; Weir, R.R.; Hill, S.; O’Reilly, J.; Kmiotek, D.; Deitrich, C.; Mackle, E.M.; Fitzgerald, E.; et al. The Effect of Processing and Seasonality on the Iodine and Selenium Concentration of Cow’s Milk Produced in Northern Ireland (NI): Implications for Population Dietary Intake. Nutrients 2018, 10, 287. [Google Scholar] [CrossRef] [Green Version]
- Mullan, K.; Hamill, L.; Doolan, K.; Young, I.; Smyth, P.; Flynn, A.; Walton, J.; Meharg, A.A.; Carey, M.; McKernan, C.; et al. Iodine Status of Teenage Girls on the Island of Ireland. Eur. J. Nutr. 2020, 59, 1859–1867. [Google Scholar] [CrossRef]
- Coneyworth, L.J.; Coulthard, L.C.H.A.; Bailey, E.H.; Young, S.D.; Stubberfield, J.; Parsons, L.; Saunders, N.; Watson, E.; Homer, E.M.; Welham, S.J.M. Geographical and Seasonal Variation in Iodine Content of Cow’s Milk in the UK and Consequences for the Consumer’s Supply. J. Trace Elem. Med. Biol. 2020, 59, 126453. [Google Scholar] [CrossRef]
- Hejtmánková, A.; Kuklík, L.; Trnková, E.; Dragounová, H. Iodine Concentrations in Cow’s Milk in Central and Northern Bohemia. Czech J. Anim. Sci. 2006, 51, 189–195. [Google Scholar] [CrossRef]
- Stimec, M.; Kobe, H.; Smole, K.; Kotnik, P.; Sirca-Campa, A.; Zupancic, M.; Battelino, T.; Krzisnik, C.; Fidler Mis, N. Adequate Iodine Intake of Slovenian Adolescents Is Primarily Attributed to Excessive Salt Intake. Nutr. Res. 2009, 29, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.A.T.; Schoen, S.A.; Salmon, G.D.; Young, B.; Johnson, R.D.; Marts, R.W. Composition of Core Foods of the U.S. Food Supply, 1982-1991: III.; Copper, Manganese, Selenium, and Iodine. J. Food Compos. Anal. 1995, 8, 171–217. [Google Scholar] [CrossRef]
- Food Nutrient Database. Available online: https://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/foodnutrient/Pages/default.aspx (accessed on 20 January 2021).
- Fordyce, F.M. Database of the Iodine Content of Food and Diets Populated with Data from Published Literature. Available online: http://nora.nerc.ac.uk/id/eprint/8354/ (accessed on 20 January 2021).
- Gandhi, A.P. Salt-Restriction and Adequate Iodine Consumption: Dual Burden or Twin-Opportunity? Natl. Med. J. India 2019, 32, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Szybiński, Z.; Jarosz, M.; Hubalewska-Dydejczyk, A.; Stolarz-Skrzypek, K.; Kawecka-Jaszcz, K.; Traczyk, I.; Stoś, K. Iodine-Deficiency Prophylaxis and the Restriction of Salt Consumption-a 21st Century Challenge. Endokrynol. Pol. 2010, 61, 135–140. [Google Scholar]
- WHO. Guideline: Fortification of Food-Grade Salt with Iodine for the Prevention and Control of Iodine Deficiency Disorders; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Eveleigh, E.R.; Coneyworth, L.J.; Avery, A.; Welham, S.J.M. Vegans, Vegetarians, and Omnivores: How Does Dietary Choice Influence Iodine Intake? A Systematic Review. Nutrients 2020, 12, 1606. [Google Scholar] [CrossRef]
- Rogerson, D. Vegan Diets: Practical Advice for Athletes and Exercisers. J. Int. Soc. Sports Nutr. 2017, 14, 36. [Google Scholar] [CrossRef] [Green Version]
- Yeliosof, O.; Silverman, L.A. Veganism as a Cause of Iodine Deficient Hypothyroidism. J. Pediatr. Endocrinol. Metab. 2018, 31, 91–94. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otun, J.; Sahebkar, A.; Östlundh, L.; Atkin, S.L.; Sathyapalan, T. Systematic Review and Meta-Analysis on the Effect of Soy on Thyroid Function. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Dennis, M.; Lee, A.R.; McCarthy, T. Nutritional Considerations of the Gluten-Free Diet. Gastroenterol. Clin. North. Am. 2019, 48, 53–72. [Google Scholar] [CrossRef]
- Melini, V.; Melini, F. Gluten-Free Diet: Gaps and Needs for a Healthier Diet. Nutrients 2019, 11, 170. [Google Scholar] [CrossRef] [Green Version]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten Free Diet and Nutrient Deficiencies: A Review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef] [Green Version]
- Jahreis, G.; Hausmann, W.; Kiessling, G.; Franke, K.; Leiterer, M. Bioavailability of Iodine from Normal Diets Rich in Dairy Products-Results of Balance Studies in Women. Exp. Clin. Endocrinol. Diabetes 2001, 109, 163–167. [Google Scholar] [CrossRef] [PubMed]
- van der Reijden, O.L.; Zimmermann, M.B.; Galetti, V. Iodine in Dairy Milk: Sources, Concentrations and Importance to Human Health. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 385–395. [Google Scholar] [CrossRef]
- Bouga, M.; Lean, M.E.J.; Combet, E. Contemporary Challenges to Iodine Status and Nutrition: The Role of Foods, Dietary Recommendations, Fortification and Supplementation. Proc. Nutr. Soc. 2018, 77, 302–313. [Google Scholar] [CrossRef]
- Dahl, L.; Wik Markhus, M.; Sanchez, P.; Moe, V.; Smith, L.; Meltzer, H.; Kjellevold, M. Iodine Deficiency in a Study Population of Norwegian Pregnant Women—Results from the Little in Norway Study (LiN). Nutrients 2018, 10, 513. [Google Scholar] [CrossRef] [Green Version]
- Coudray, B. The Contribution of Dairy Products to Micronutrient Intakes in France. J. Am. Coll. Nutr. 2011, 30, 410S–414S. [Google Scholar] [CrossRef] [PubMed]
- Ovadia, Y.S.; Gefel, D.; Weizmann, N.; Raizman, M.; Goldsmith, R.; Mabjeesh, S.J.; Dahl, L.; Troen, A.M. Low Iodine Intake from Dairy Foods Despite High Milk Iodine Content in Israel. Thyroid 2018, 28, 1042–1051. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzel-Viljoen, E.; Avezum, A.; et al. Association of Dairy Intake with Cardiovascular Disease and Mortality in 21 Countries from Five Continents (PURE): A Prospective Cohort Study. Lancet 2018, 392, 2288–2297. [Google Scholar] [CrossRef]
- Rice, B.H.; Quann, E.E.; Miller, G.D. Meeting and Exceeding Dairy Recommendations: Effects of Dairy Consumption on Nutrient Intakes and Risk of Chronic Disease. Nutr. Rev. 2013, 71, 209–223. [Google Scholar] [CrossRef] [Green Version]
- Gunnarsdottir, I.; Gustavsdottir, A.G.; Steingrimsdottir, L.; Maage, A.; Johannesson, A.J.; Thorsdottir, I. Iodine Status of Pregnant Women in a Population Changing from High to Lower Fish and Milk Consumption. Public Health Nutr. 2013, 16, 325–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Condo, D.; Huyhn, D.; Anderson, A.J.; Skeaff, S.; Ryan, P.; Makrides, M.; Mühlhaüsler, B.S.; Zhou, S.J. Iodine Status of Pregnant Women in South Australia after Mandatory Iodine Fortification of Bread and the Recommendation for Iodine Supplementation. Matern. Child. Nutr. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Eastman, C.J.; Jooste, P. Current Challenges in Meeting Global Iodine Requirements. In Nestlé Nutrition Institute Workshop Series; Bhutta, Z.A., Hurrell, R.F., Rosenberg, I.H., Eds.; S. KARGER AG: Basel, Switzerland, 2012; Volume 70, pp. 147–160. ISBN 978-3-318-02111-0. [Google Scholar]
- Bath, S.C.; Hill, S.; Infante, H.G.; Elghul, S.; Nezianya, C.J.; Rayman, M.P. Iodine Concentration of Milk-Alternative Drinks Available in the UK in Comparison with Cows’ Milk. Br. J. Nutr. 2017, 118, 525–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M.B.; Crill, C.M. Iodine in Enteral and Parenteral Nutrition. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 143–158. [Google Scholar] [CrossRef]
- Guidetti, M.; Agostini, F.; Lapenna, G.; Pazzeschi, C.; Soverini, V.; Petitto, R.; Pironi, L. Iodine Nutrition in Adults on Long-Term Home Parenteral Nutrition. Nutrition 2014, 30, 1050–1054. [Google Scholar] [CrossRef]
- Zimmermann, M.B. Iodine Deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef] [Green Version]
- Vanek, V.W.; Borum, P.; Buchman, A.; Fessler, T.A.; Howard, L.; Jeejeebhoy, K.; Kochevar, M.; Shenkin, A.; Valentine, C.J.; Novel Nutrient Task Force, Parenteral Multi-Vitamin and Multi–Trace Element Working Group; et al. A.S.P.E.N. Position Paper: Recommendations for Changes in Commercially Available Parenteral Multivitamin and Multi–Trace Element Products. Nutr. Clin. Pr. 2012, 27, 440–491. [Google Scholar] [CrossRef] [PubMed]
- Willard, D.L.; Young, L.S.; He, X.; Braverman, L.E.; Pearce, E.N. Iodine Content of Enteral and Parenteral Nutrition Solutions. Endocr. Pract. 2017, 23, 775–779. [Google Scholar] [CrossRef]
- Navarro, A.M.; Suen, V.M.M.; Souza, I.M.; De Oliveira, J.E.D.; Marchini, J.S. Patients with Severe Bowel Malabsorption Do Not Have Changes in Iodine Status. Nutrition 2005, 21, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Findik, R.B.; Yilmaz, G.; Celik, H.T.; Yilmaz, F.M.; Hamurcu, U.; Karakaya, J. Effect of Povidone Iodine on Thyroid Functions and Urine Iodine Levels in Caesarean Operations. J. Matern. Fetal Neonatal. Med. 2014, 27, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Tahirović, H.; Toromanović, A.; Grbić, S.; Bogdanović, G.; Fatušić, Z.; Gnat, D. Maternal and Neonatal Urinary Iodine Excretion and Neonatal TSH in Relation to Use of Antiseptic During Caesarean Section in an Iodine Sufficient Area. J. Pediatric Endocrinol. Metab. 2009, 22, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Kurtoglu, S.; Bastug, O.; Daar, G.; Halis, H.; Korkmaz, L.; Memur, S.; Korkut, S.; Gunes, T.; Ozturk, M.A. Effect of Iodine Loading on the Thyroid Hormone Level of Newborns Living in Kayseri Province. Am. J. Perinatol. 2014, 31, 1087–1092. [Google Scholar] [CrossRef]
- Belfort, M.B.; Pearce, E.N.; Braverman, L.E.; He, X.; Brown, R.S. Low Iodine Content in the Diets of Hospitalized Preterm Infants. J. Clin. Endocrinol. Metab. 2012, 97, E632–E636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genoni, A.; Lyons-Wall, P.; Lo, J.; Devine, A. Cardiovascular, Metabolic Effects and Dietary Composition of Ad-Libitum Paleolithic vs. Australian Guide to Healthy Eating Diets: A 4-Week Randomised Trial. Nutrients 2016, 8, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, K.; Schöne, F.; Berk, A.; Leiterer, M.; Flachowsky, G. Influence of Dietary Iodine on the Iodine Content of Pork and the Distribution of the Trace Element in the Body. Eur. J. Nutr. 2008, 47, 40–46. [Google Scholar] [CrossRef]
- Manousou, S.; Stål, M.; Larsson, C.; Mellberg, C.; Lindahl, B.; Eggertsen, R.; Hulthén, L.; Olsson, T.; Ryberg, M.; Sandberg, S.; et al. A Paleolithic-Type Diet Results in Iodine Deficiency: A 2-Year Randomized Trial in Postmenopausal Obese Women. Eur. J. Clin. Nutr. 2018, 72, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Churuangsuk, C.; Griffiths, D.; Lean, M.E.J.; Combet, E. Impacts of Carbohydrate-Restricted Diets on Micronutrient Intakes and Status: A Systematic Review. Obes. Rev. 2019, 20, 1132–1147. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.C.Y.; Buyken, A.E.; Brand-Miller, J.C.; Flood, V.M. The Link between Dietary Glycemic Index and Nutrient Adequacy. Am. J. Clin. Nutr. 2012, 95, 694–702. [Google Scholar] [CrossRef] [Green Version]
- Kopp, W. Nutrition, Evolution and Thyroid Hormone Levels–a Link to Iodine Deficiency Disorders? Med. Hypotheses 2004, 62, 871–875. [Google Scholar] [CrossRef]
- Siniorakis, E.; Arvanitakis, S.; Zarreas, E.; Saridakis, M.; Balanis, A.; Tzevelekos, P.; Bokos, G.; Limberi, S. Mediterranean Diet: Natural Salicylates and Other Secrets of the Pyramid. Int. J. Cardiol. 2013, 166, 538–539. [Google Scholar] [CrossRef]
- Chiang, H.-L.; Venter, C.; Syue, P.-C.; Ku, K.-L.; Wu, C.-H. Which Fruits and Vegetables Should Be Excluded from a Low-Salicylate Diet? An Analysis of Salicylic Acid in Foodstuffs in Taiwan. Int. Arch. Allergy Immunol. 2018, 176, 198–204. [Google Scholar] [CrossRef]
- Szczuko, M.; Romaniuk, R. Dieta niskosalicylanowa a możliwość występowania niedoborów składników pokarmowych. Pomeranian J. Life Sci. 2017, 62. [Google Scholar] [CrossRef] [Green Version]
- Leniszewski, S.; Mauseth, R. Goiter and Multiple Food Allergies. Int. J. Pediatric Endocrinol. 2009, 2009, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Skodje, G.I.; Minelle, I.H.; Rolfsen, K.L.; Iacovou, M.; Lundin, K.E.A.; Veierød, M.B.; Henriksen, C. Dietary and Symptom Assessment in Adults with Self-Reported Non-Coeliac Gluten Sensitivity. Clin. Nutr. Espen 2019, 31, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, T.; Plumb, E.; Callaghan, J.; Jackson, M.; Michaelis, L. Dietary Restriction Causing Iodine-Deficient Goitre. Arch. Dis. Child. 2015, 100, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Kawashima, A.; Ishido, Y.; Yoshihara, A.; Oda, K.; Hiroi, N.; Ito, T.; Ishii, N.; Suzuki, K. Iodine Excess as an Environmental Risk Factor for Autoimmune Thyroid Disease. Int. J. Mol. Sci. 2014, 15, 12895–12912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teas, J.; Pino, S.; Critchley, A.; Braverman, L.E. Variability of Iodine Content in Common Commercially Available Edible Seaweeds. Thyroid 2004, 14, 836–841. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Iodine. Efsa J. 2014, 12, 3660. [Google Scholar] [CrossRef] [Green Version]
- Scientific Committee on Food Opinion of the Scientific Committee on Food on the Tolerable Upper Intake Level of Iodine. 2002.
- Leung, A.M.; Braverman, L.E. Iodine-Induced Thyroid Dysfunction. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Farebrother, J.; Zimmermann, M.B.; Andersson, M. Excess Iodine Intake: Sources, Assessment, and Effects on Thyroid Function. Ann. N. Y. Acad. Sci. 2019, 1446, 44–65. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and Benefits of Consuming Edible Seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, M.; Delange, F. Iodine Supplementation of Pregnant Women in Europe: A Review and Recommendations. Eur. J. Clin. Nutr. 2004, 58, 979–984. [Google Scholar] [CrossRef] [Green Version]
- Prete, A.; Paragliola, R.M.; Corsello, S.M. Iodine Supplementation: Usage “with a Grain of Salt”. Int. J. Endocrinol. 2015, 2015, 312305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gietka-Czernel, M.; Glinicki, P. Subclinical Hypothyroidism in Pregnancy: Controversies on Diagnosis and Treatment. Pol. Arch. Intern. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Han, C.; Li, C.; Mao, J.; Wang, W.; Xie, X.; Li, C.; Xu, B.; Meng, T.; Du, J.; et al. Optimal and Safe Upper Limits of Iodine Intake for Early Pregnancy in Iodine-Sufficient Regions: A Cross-Sectional Study of 7190 Pregnant Women in China. J. Clin. Endocrinol. Metab. 2015, 100, 1630–1638. [Google Scholar] [CrossRef] [Green Version]
- Teng, W.; Shan, Z.; Teng, X.; Guan, H.; Li, Y.; Teng, D.; Jin, Y.; Yu, X.; Fan, C.; Chong, W.; et al. Effect of Iodine Intake on Thyroid Diseases in China. N. Engl. J. Med. 2006, 354, 2783–2793. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Gosnell, J.E.; Roman, S.A. Geographic Influences in the Global Rise of Thyroid Cancer. Nat. Rev. Endocrinol. 2020, 16, 17–29. [Google Scholar] [CrossRef]
- Żach, M.; Kryjan, K.; Ambroziak, U.; Witkowska, M.; Karpiński, G.; Opolski, G.; Bednarczuk, T. Hyperthyroidism after Iodine-Containing Contrast Agent Administration. Kardiol. Pol. 2013, 71, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.; Chaikoff, I.L. Plasma Inorganic Iodide as a Homeostatic Regulator of Thyroid Function. J. Biol. Chem. 1948, 174, 555–564. [Google Scholar] [CrossRef]
- Gardner, D.G. Nadczynność tarczycy wywołana przyjmowaniem amiodaronu. In Endokrynologia Ogólna I Kliniczna Greenspana; Gardner, D.G., Shoback, D., Eds.; Czelej sp. z o.o.: Lublin, Poland, 2011; pp. 915–916. [Google Scholar]
- Rah, J.H.; Anas, A.M.; Chakrabarty, A.; Sankar, R.; Pandav, C.S.; Aguayo, V.M. Towards Universal Salt Iodisation in India: Achievements, Challenges and Future Actions. Matern. Child. Nutr. 2013, 11, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Codling, K.; Chang, S.; Zhang, S.; Shen, H.; Su, X.; Chen, Z.; Scherpbier, R.W.; Yan, J. Eliminating Iodine Deficiency in China: Achievements, Challenges and Global Implications. Nutrients 2017, 9, 361. [Google Scholar] [CrossRef]


| Group | RDA (Recommended Dietary Allowances), (μg) | Adequate Intake (AI), (μg) |
|---|---|---|
| 0–6 months | 110 | |
| 7–12 months | 130 | |
| 1–3 years | 90 | |
| 4–8 years | 90 | |
| 9–13 years | 120 | |
| 14–18 years | 150 | |
| ≥19 years | 150 | |
| Pregnancy | 220 | |
| Breastfeeding women | 290 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krela-Kaźmierczak, I.; Czarnywojtek, A.; Skoracka, K.; Rychter, A.M.; Ratajczak, A.E.; Szymczak-Tomczak, A.; Ruchała, M.; Dobrowolska, A. Is There an Ideal Diet to Protect against Iodine Deficiency? Nutrients 2021, 13, 513. https://doi.org/10.3390/nu13020513
Krela-Kaźmierczak I, Czarnywojtek A, Skoracka K, Rychter AM, Ratajczak AE, Szymczak-Tomczak A, Ruchała M, Dobrowolska A. Is There an Ideal Diet to Protect against Iodine Deficiency? Nutrients. 2021; 13(2):513. https://doi.org/10.3390/nu13020513
Chicago/Turabian StyleKrela-Kaźmierczak, Iwona, Agata Czarnywojtek, Kinga Skoracka, Anna Maria Rychter, Alicja Ewa Ratajczak, Aleksandra Szymczak-Tomczak, Marek Ruchała, and Agnieszka Dobrowolska. 2021. "Is There an Ideal Diet to Protect against Iodine Deficiency?" Nutrients 13, no. 2: 513. https://doi.org/10.3390/nu13020513