Abstract
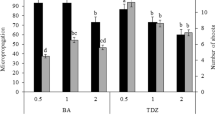
An effective protocol was developed for in vitro regeneration of the Melothria maderaspatana via indirect organogenesis in liquid and solid culture systems. Organogenesis was achieved from liquid culture calluses derived from leaf and petiole explants of mature plants. Organogenic calluses (98.2 ± 0.36 and 94.8 ± 0.71%) were induced from both leaf and petiole explants on Murashige and Skoog (MS) liquid medium containing 6.0 µM 2,4-dichlorophenoxyacetic acid (2,4-D) and 0.5 µM thidiazuron (TDZ); and 6.0 µM 2,4-D and 1.0 µM benzyladenine (BA) combinations, respectively. Adventitious shoot regeneration (68.2 ± 0.06 shoots per explant) was achieved on MS medium supplemented with 2.0 µM BA, 4.0 µM TDZ, 10% v/v coconut water and 0.06 mM glutamine from leaf-derived calluses. Petiole-derived calluses produced adventitious shoots (45.4 ± 0.09 shoots per explant) on MS medium fortified with 2.0 µM BA, 4.0 µM TDZ, 10% v/v coconut water, and 0.08 mM glutamine. Elongation of shoots occurred in MS medium with 2.0 µM gibberellic acid (GA3). Regenerated shoots (2–3 cm in length) rooted (74.2 ± 0.38%) and hardened (85 ± 1.24%) when they were transferred to 1/2-MS medium supplemented with 3.0 µM indole-3-butyric acid (IBA) followed by garden soil, vermiculate, and sand (2:1:1 ratio) mixture. The elongated shoots (4–5 cm in length) were exposed simultaneously for rooting as well as hardening (100%) in moistened [(1/8-MS basal salt solution with 5 µM IBA and 100 mg l−1 Bavistin® (BVN)] garden soil, vermiculate, and sand (2:1:1 ratio) mixture. Subsequently, the plants were successfully established in the field. The survival percentage differed with seasonal variations.

Similar content being viewed by others
References
Amutha S.; Ganapathi A.; Muruganantham M. In vitro organogenesis and plant formation in Vigna radiata (L.) Wilczek. Plant Cell, Tissue Organ Cult. 72: 203–207; 2003. doi:10.1023/A:1022266110750.
Anis M.; Faisal M. In vitro regeneration and mass multiplication of Psoralea corylifolia—an endangered medicinal plant. Indian J. Biotechnol. 4: 261–264; 2005.
Baskaran P.; Jayabalan N. An efficient micropropagation system for Eclipta alba–a valuable medicinal herb. In Vitro Cell. Dev. Biol., Plant 41: 532–539; 2005. doi:10.1079/IVP2005667.
Baskaran P.; Jayabalan N. Rapid micropropagation of Psoralea corylifolia L. using nodal explants cultured in organic additive-supplemented medium. J. Hortic. Sci. Biotechnol. 82: 908–913; 2007.
Delp C. J. Benzimidazole and related fungicides. In: Lyr H. (ed) Modern selective fungicides. VEB Gustav Fischer Verlag, London, pp 233–244; 1987.
Gamborg O. L.; Miller R. A.; Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res 50: 151–158; 1968. doi:10.1016/0014–4827(68)90403–5.
Garcia P. C.; Rivero R. M.; Ruiz J. M.; Romero L. The role of fungicides in the physiology of higher plants: implications for defense responses. Bot. Rev 69: 162–172; 2003. doi:10.1663/0006-8101(2003)069[0162:TROFIT]2.0.CO;2.
Gordon S. A. Occurrence, formation and inactivation of auxin. Ann. Rev. Plant Physiol 5: 341–378; 1954. doi:10.1146/annurev.pp.05.060154.002013.
Handley L. W.; Chambliss O. L. In vitro propagation of Cucumis sativus L. Sci. Hortic 14: 22–23; 1979.
Huetteman C. A.; Preece J. E. Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell, Tissue Organ Cult 33: 105–119; 1993. doi:10.1007/BF01983223.
Hussein A. S. M.; Kingston D. G. I. Screening of plants used in Sudan folk medicine for anticancer activity (II). Fitoterapia 53: 119–123; 1982.
Jayatilaka K. A. P. W.; Thabrew M. I.; Pathirana C.; De Silva D. G. H.; Perera D. J. B. An evaluation of the potency of Osbeckia octandra and Melothria maderaspatana as antihepatotoxic agents. Planta Med 55: 137–139; 1989. doi:10.1055/s-2006–961906.
Kathal R.; Bhatnagar S. P.; Bhojwani S. S. Regeneration of plants from leaf explants of Cucumis melo cv. Pusa sharbati. Plant Cell Rep 7: 449–451; 1988.
Locy R. D.; Wehner T. C. Cucumber shoot tip growth on 9 nitrogen sources in in vitro culture. Cucurbits Genet. Coop. Rep 5: 10–11; 1982.
Meira Z. Bioreactor technology for plant micropropagation. Hortic. Rev 24: 1–30; 2000.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15: 473–497; 1962. doi:10.1111/j.1399-3054.1962.tb08052.x.
Nabi S. A.; Rashid M. M.; Al-Amin M.; Rasul M. G. Organogenesis in teasle gourd (Momordica dioica Roxb.). Plant Tissue Cult 12: 173–180; 2002.
Nugent G.; Wardley-Richardson T.; Lu C. L. Plant regeneration from stem and petal of carnation (Dianthus caryophyllus L.). Plant Cell Rep 10: 477–480; 1991. doi:10.1007/BF00233819.
Punja Z. K.; Abbas N.; Sarmento G. G.; Tang F. A. Regeneration of Cucumis sativus vars, sativus and hardwickii, C. melo and C. metuliferous from explants through somatic embryogenesis and organogenesis. Plant Cell, Tissue Organ Cult. 21: 93–102; 1990. doi:10.1007/BF00033427.
Ramakrishanamacharya C. H.; Krishnaswamy M. R.; Bhima Rao R.; Viswanathan S. Anti-inflammatory efficacy of Melothria madraspatana in active rheumatoid arthritis [1]. Clin. Rheumatol 15: 214–215; 1996. doi:10.1007/BF02230346.
Sahoo Y.; Chand P. K. Micropropagation of vitex negundo L. a woody aromatic medicinal shrub, through high frequency axillary shoot proliferation. Plant Cell Rep 18: 301–307; 1998. doi:10.1007/s002990050576.
Selvaraj N.; Vasudevan A.; Manickavasagam M.; Ganapathi A. In vitro organogenesis and plant formation in cucumber. Biol. Plant 50: 123–126; 2006. doi:10.1007/s10535-005-0085-7.
Seo S. H.; Bai D. G.; Park H. Y. High frequency shoot regeneration from leaf explants of cucumber. J. Plant Biotechnol 21: 51–54; 2000.
Sinha B. N.; Sasmal D.; Basu S. P. Pharmacological studies on Melothria maderaspatana. Fitoterapia 68: 75–78; 1997.
Skirvin R. M.; Chu M. C.; Rukan H. An improved medium for the in vitro rooting of Harbrite peach. Proc. III State Hort. Soc 113: 30–38; 1980.
Tejavathi D. H.; Shailaja K. S. Regeneration of plants from the cultures of Bacopa monnieri (L.). Pennell Phytomorphol 49: 447–452; 1999.
Thabrew M. I.; Gove C. D.; Hughes R. D.; McFarlane I. G.; Williams R. Protection against galactosamine and tert-butyl hydroperoxide induced hepatocyte damage by Melothria maderaspatana extract. Phytotherapy Res. 9: 513–517; 1995. doi:10.1002/ptr.2650090710.
Thabrew M. I.; Jayatilaka K. A. P. W.; Perera D. J. B. Evaluation of the efficacy of Melothria maderaspatana in the alleviation of carbon tetrachloride-induced liver dysfunction. J. Ethnopharmacol 23: 305–312; 1988. doi:10.1016/0378-8741(88)90010-4.
Tisserat B.; Murashige T. Probable identity of substances in Citrus that repress asexual embryogenesis. In Vitro 13: 785–789; 1977.
Tremblay F. M.; Lalonde M. Requirements for in vitro propagation of seven nitrogen- fixing Alnus species. Plant Cell, Tissue Organ Cult. 3: 189–199; 1984. doi:10.1007/BF00040337.
Tripathi R. K.; Ram S. Induction of growth and differentiation of carrot callus cultures by carbendazim and benzimidazole. Indian J. Exp. Biol 20: 674–677; 1982.
Vaidyaratnam P. S. V. Indian medicinal plants. Orient Longman Limited, Madras, India; 1995.
Vasudevan A.; Selvaraj N.; Ganapathi A.; Kasthurirengan S.; Ramesh Anbazhagan V.; Manickavasagam M. Glutamine: a suitable nitrogen source for enhanced shoot multiplication in Cucumis sativus. Biol. Plant 48: 125–128; 2004. doi:10.1023/B:BIOP.0000024288.82679.50.
Wang H. C.; Chen J. T.; Wu S. P.; Lin M. C.; Chang W. C. Plant regeneration through shoot formation from callus of Areca catechu L. Plant Cell, Tissue Organ Cult 75: 95–98; 2003. doi:10.1023/A:1024649428393.
Acknowledgements
We are grateful to Director Dr. Rev. Fr. John Britto, Rapinat Herbarium, for selection of this species. The author also wishes to thank University of Bharathidasan Research Fund for financial support of this work, G. G. Gideon for suggestions and P. Sasikumar for the valuable help in plant collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: E. Bunn
Rights and permissions
About this article
Cite this article
Baskaran, P., Velayutham, P. & Jayabalan, N. In vitro regeneration of Melothria maderaspatana via indirect organogenesis. In Vitro Cell.Dev.Biol.-Plant 45, 407–413 (2009). https://doi.org/10.1007/s11627-008-9172-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-008-9172-8